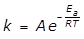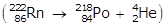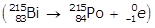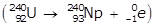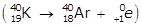Preparation Manual
Section 4: Sample Selected-Response Questions Chemistry 7–12 (240)
Expand All Answers | Collapse All Answers
This section presents some sample exam questions for you to review as part of your preparation for the exam. To demonstrate how each competency may be assessed, sample questions are accompanied by the competency that they measure. While studying, you may wish to read the competency before and after you consider each sample question. Please note that the competency statements do not appear on the actual exam.
For each sample exam question, there is a correct answer and a rationale for each answer option. The sample questions are included to illustrate the formats and types of questions you will see on the exam; however, your performance on the sample questions should not be viewed as a predictor of your performance on the actual exam.
The following reference materials will be available to you during the exam:
Domain I—Scientific Inquiry and Processes
Competency 001—The teacher understands how to select and manage learning activities to ensure the safety of all students and the correct use and care of natural resources, materials, equipment and technologies.
1. The volume of a liquid sample can be measured and reported as 18.00 mL by using which of the following pieces of glassware?
- 25 mL graduated cylinder
- 25 mL beaker
- 50 mL buret
- 50 mL Erlenmeyer flask
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because the smallest divisions on a scale of a 50 mL buret are typically 0.1 mL. Thus, the volume of the sample can be estimated to two decimal places: 18.00 ± 0.01 mL. Option A is incorrect because the smallest divisions on a scale of a 25 mL graduated cylinder are typically 1 mL. Thus, the volume of the sample can be estimated to one decimal place only: 18.0 ± 0.1 mL. Option B is incorrect because the smallest divisions on a scale of a 25 mL beaker are 5 mL or greater. Thus, the volume of the sample can be estimated to be between 15 and 20 mL. Option D is incorrect because the smallest divisions on a scale of a 50 mL Erlenmeyer flask are typically 10 mL. Thus, the volume of the sample can be estimated to be between 10 and 20 mL.
2. Which of the following is safety equipment that can be found in a high school chemistry lab?
- Bunsen burner
- Eyewash station
- Barometer
- Glass mercury thermometer
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because an eyewash station is used to flush the eyes when liquids have been splashed or sprayed into a person's eyes. Option A is incorrect because a Bunsen burner is used to heat some materials in the lab and must be used with care. Option C is incorrect because a barometer is used to measure atmospheric pressure. Option D is incorrect because a glass mercury thermometer can pose a significant hazard due to possible broken glass and mercury exposure.
3. A shipment of chemicals is delivered to a high school chemistry lab. Of the following, the most appropriate way to store the substances is to organize them by
- the alphabetical names of the substances.
- the concentrations of the solutions.
- solids or liquids.
- the chemical properties of the substances.
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because substances that are stored near each other might react chemically with each other and create safety concerns. Options A, B, and C are incorrect because they are organizational concepts that are not as important as the possibility of chemical reactions between the various substances.
Competency 002—The teacher understands the nature of science and the process of scientific inquiry.
4. Of the following, which is most appropriate to do after forming a hypothesis?
- Organize data
- Draw conclusions
- Propose a theory
- Test the hypothesis
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because a hypothesis must be tested in order to support or disprove it. Option A is incorrect because the testing data have not yet been produced, and any preliminary observational data available should have been organized and analyzed in the process of developing a hypothesis. Option B is incorrect because the hypothesis has not yet been tested, so there are not sufficient data for analyzing and drawing conclusions. Option C is incorrect because a theory cannot be developed until enough testing has been done to produce data that support the hypothesis.
5. Students conducted an activity to determine the acidity of several liquid products commonly used in homes. They added several drops of red cabbage juice to samples of the substances and recorded the resulting colors. The activity is best described as which of the following?
- Statistical analysis
- Forensic analysis
- Descriptive study
- Synthesis experiment
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because a descriptive study indicates characteristics, not causes. Option A is incorrect because no numerical data were collected. Option B is incorrect because only observations were recorded and no analysis was done. Option D is incorrect because no synthesis reactions took place.
Competency 003—The teacher understands the role of mathematics and unifying concepts common to all sciences.
6. Which of the following is an example of a model?
- The equation PV = nRT.
- A liquid has a green color.
- Chemistry is a vital part of biology.
- When 12 g of carbon completely reacted with oxygen, it formed 44 grams of carbon dioxide.
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because the equation represents a mathematical model of the behavior of an ideal gas in which intermolecular attractions and gas particle volume are accounted for. Option B is incorrect because the color of the liquid is an observation. Option C is incorrect because although chemistry is very much a part of biology, this fact does not represent a model. Option D is incorrect because the quantitative analysis of the carbon reaction is an observation based on analysis of data and does not represent a model.
7. A linear measurement of 156.020 mm is made. Which of the following is the best representation in scientific notation that indicates the correct number of significant figures?
- 1.56020 times 10 to the power of 5 mm
- 1.56020 times 10 squared mm
- 1.5602 times 10 squared mm
- 1.56 times 10 squared mm
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because the number 156.020 is represented by two powers of ten left paren 10 superscript 2 right paren and indicates six significant figures. Option A is incorrect because it incorrectly indicates five powers of ten left paren 10 superscript 5 right paren, even though it does include all six significant figures. Option C is incorrect because although it correctly indicates two powers of ten left paren 10 superscript 2 right paren, it does not include all six significant figures; it includes only five. Option D is incorrect because although it correctly indicates two powers of ten left paren 10 superscript 2 right paren, it does not include all six significant figures; it includes only three.
Competency 004—The teacher understands the history of science, how science impacts the daily lives of students and how science interacts with and influences personal and societal decisions.
8. Of the following, which contributes the most to water pollution in streams near mountains?
- Nuclear power plants
- Mine drainage
- Carbon dioxide emissions from gas-powered automobiles
- Oil-well drilling
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because acid and metal ion mine-drainage from abandoned coal mines has a significant impact on many streams in mountainous coal-mining regions. Option A is incorrect because although nuclear power plants can contribute to thermal pollution, plants are typically located near rivers or oceans, not in mountain regions near streams. Radioactive emissions are not common and are not the major source of water pollution in mountain streams. Option C is incorrect because carbon dioxide emissions can lead to a minor amount of dissolved carbon dioxide (carbonic acid), but the level is not considered significant. Option D is incorrect because oil-well drilling is not typically done in areas that could affect streams in mountainous regions.
9. Which of the following scientists is particularly noted for his or her contribution to radiochemistry?
- Robert Boyle
- Marie Curie
- Dmitri Mendeleev
- John Dalton
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because Marie Curie did extensive work in isolating radioactive isotopes, and she discovered two radioactive elements. Option A is incorrect because Robert Boyle is most known for his discovery, Boyle's law, which indicates that the volume of a gas is inversely proportional to the absolute pressure of the gas if the temperature is kept constant in a closed system. Option C is incorrect because Dmitri Mendeleev is most known for the development of a periodic table. Option D is incorrect because John Dalton is most known for his atomic theory, which describes some basic principles of atoms and how they combine to form compounds, but it does not involve radioactivity.
Domain II—Matter and Energy
Competency 005—The teacher understands the characteristics of matter.
10. Of the following, which is an example of a physical change only?
- Snow sublimating in the Arctic
- An iron nail rusting
- A candle burning
- A lead storage battery recharging
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because snow left paren H subscript 2 O) changes state from solid to gas, which is a physical change, and no chemical changes occur. Option B is incorrect because Fe in the iron nail reacts with O subscript 2 to form FeO subscript 2, which is a chemical change. Option C is incorrect because a candlewick reacts with oxygen in the flame, and a combustion reaction occurs, which is a chemical change. Option D is incorrect because recharging a lead storage battery involves an electrochemical reaction.
11. Which of the following is true about elements?
- When a chemical reaction takes place, the particles in the nucleus of the atoms are rearranged.
- Isotopes are different forms of an element in which the atoms have a different number of protons.
- All atoms of the element carbon have the same mass.
- Compounds consist of two or more different kinds of elements.
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because compounds are composed of two or more elements. Option A is incorrect because chemical reactions do not involve changes in the nucleus. Option B is incorrect because isotopes of an element have the same number of protons but a different number of neutrons. Option C is incorrect because carbon has isotopes that have atoms with a different number of neutrons and hence a different mass even though they have the same number of protons and electrons. For example, all carbon atoms have 6 protons and 6 electrons, but carbon-12 has 6 neutrons and carbon-13 has 7 neutrons.
Competency 006—The teacher understands the structure and characteristics of atoms.
12. Based on the periodic table, which of the following atoms has the highest first-ionization energy?
- Ca
- Ni
- Br
- I
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because, based on periodic trends, the first-ionization energy increases from left to right in a row on the periodic table and decreases from top to bottom of a column. Br is farther to the right than Ni and Ca in a row and is above I in a column. Option A is incorrect because on the periodic table Ca is farther to the left on a row than Br. Option B is incorrect because on the periodic table Ni is farther to the left on a row than Br. Option D is incorrect because on the periodic table I is below Br in a column.
13. Which of the following is the ground-state electron configuration for Se?
- 1s squared 2s squared 2p to the power of 6 3 s squared 3p to the power of 6 4s squared 4p to the power of 4
- 1s squared 2s squared 2p to the power of 6 3s squared 3p to the power of 6 4s squared 4p to the power of 6 5s squared 5p to the power of 6
- 1s squared 2s squared 2p to the power of 6 3s squared 3p to the power of 6 3d to the power of 10 4s squared 4p to the power of 4
- 1s squared 2s squared 2p to the power of 6 3s squared 3p to the power of 6 4s squared 4p to the power of 4 4d to the power of 10
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because it contains the correct number of electrons and correctly indicates the electron configuration according to the Aufbau principle. Option A is incorrect because it contains 24 electrons, whereas Se has 34 electrons. Option B is incorrect because it contains the correct number of electrons, but it is missing the 3d electrons. Option D is incorrect because it contains the correct number of electrons, but it has 4d electrons, not 3d electrons.
14. When hydrogen atoms in excited electronic states undergo transitions to lower electronic states, they emit specific wavelengths of infrared, visible, or ultraviolet light. Ultraviolet light will be emitted when which of the following electronic energy level transitions occur? (n is the principle quantum number for an electronic energy level.)
- n = 3 to n = 2
- n = 4 to n = 1
- n = 4 to n = 3
- n = 5 to n = 4
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because ultraviolet light has a shorter wavelength and a higher energy than visible or infrared light. Ultraviolet emissions occur for transitions from higher energy levels to the n = 1 level. Visible emissions occur from transitions from higher energy levels to the n = 2 level. Infrared emissions occur for transitions from higher energy levels to the n = 3 level. The electronic energy levels get closer together as n increases as can be seen from the following equation.
delta E equals R subscript H left paren start fraction 1 over n subscript initial squared end fraction minus start fraction 1 over n subscript final squared end fraction right paren
Options A, C, and D are incorrect because the energies involved in those transitions are smaller than for n = 4 to n = 1.
Competency 007—The teacher understands the properties of gases.
15. When the temperature increases in a sample of gas molecules in a closed container of fixed volume, which of the following changes takes place?
- A decrease in the frequency of collisions between molecules
- An increase in the volume of each molecule
- An increase in the average attractive forces between molecules
- An increase in the average speed of the molecules
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because as temperature increases, the average speed of the molecules in the sample will increase. Option A is incorrect because the frequency of collisions will increase, not decrease. Option B is incorrect because the actual volume of each molecule does not change with temperature. Option C is incorrect because the attractive forces are dependent on the electrostatic nature of the molecules and the distance between the molecules. As temperature increases, the electrostatic properties of each molecule do not change, and the sample is in a fixed-volume container, so the average distance between molecules will not change. Hence, there is no change in the average attractive forces between molecules.
16. A gas sample at 300.0 K and 1.0 atm is allowed to expand into an adjoining vessel until the volume is doubled. The gas is then heated, causing the temperature to increase to 900.0 K. What is the final pressure of the gas sample?
- 1.0 atm 1.0 atmosphere
- 1.5 atm 1.5 atmospheres
- 2.0 atm 2.0 atmospheres
- 6.0 atm 6.0 atmospheres
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because pressure of a fixed number of moles of gas is inversely proportional to volume and directly proportional to temperature. left paren P subscript 2 equals P subscript 1 times start fraction V subscript 1 over V subscript 2 end fraction times start fraction T subscript 2 over T subscript 1 right paren. Hence, P subscript 2 equals 1.5 atm, calculated as follows: left paren P subscript 2 equals 1 atm times 1 half times 900 K over 300 K right paren equals 1.5 atm. Option A is incorrect because pressure is not 1.0 atm. Option C is incorrect because pressure is not 2.0 atm. Option D is incorrect because pressure is not 6.0 atm.
Competency 008—The teacher understands properties and characteristics of ionic and covalent bonds.
17. Which of the following compounds has predominately covalent bonding?
- CaCl subscript 2
- AlCl subscript 3
- CH subscript 2 Cl subscript 2
- Na subscript 2 O
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because in CH subscript 2 Cl subscript 2 molecules both the C single bond H and the C single bond Cl bonds are covalent. Option A is incorrect because the compound CaCl subscript 2 has ionic bonding between metallic Ca 2 positive ions and nonmetallic Cl negative ions. Option B is incorrect because the compound AlCl subscript 3 has ionic bonding between metallic Al 3 positive ions and nonmetallic Cl negative ions. Option D is incorrect because the compound Na subscript 2 O has ionic bonding between metallic Na positive ions and nonmetallic O 2 negative ions.
18. Which of the following molecules has trigonal planar geometry?
- NH subscript 3
- H subscript 2 O
- BH subscript 3
- CO subscript 2
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because the molecule BH subscript 3 has trigonal planar geometry due to having three bonding pairs but no electron pairs around the central atom (boron). Based on valence-shell-electron-pair-repulsion (VSEPR) theory, this configuration has the minimum repulsion. Option A is incorrect because the NH3 molecule has trigonal pyramidal geometry. Option B is incorrect because the molecule H subscript 2 O has bent geometry. Option D is incorrect because the molecule CO subscript 2 has linear geometry.
Competency 009—The teacher understands and interprets chemical notation and chemical equations.
19. How many oxygen atoms are in 2.0 mol of NaNO subscript 3?
- 3
- 6
- 1.8 times 10 to the power of 24
- 3.6 times 10 to the power of 24
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because there are three oxygen atoms in the formula, there are 2 moles of the compound, and there is a total of 2 times 3 times 6.02 times 10 to the power of 23 equals 3.6 times 10 to the power of 24 oxygen atoms. Option A is incorrect because there are three oxygen atoms in the formula but many more in 2.0 mol of NaNO subscript 3. Option B is incorrect because there are six oxygen atoms in two units of the formula but many more in 2.0 mol of NaNO subscript 3. Option C is incorrect because there are 1.8 times 10 to the power of 24 oxygen atoms in 1.0 mol of NaNO subscript 3, but there is twice that amount in 2.0 mol of NaNO subscript 3.
20. Which of the following is the balanced equation for the displacement reaction of potassium with aluminum nitrate?
- 3 K plus A L left paren N O subscript 3 right paren subscript 3 which becomes 3 K N O subscript 3 plus A L
- K plus A L N O subscript 3 which becomes K N O subscript 3 plus A L
- 3 K plus A L N O subscript 3 which becomes K subscript 3 N plus A L O subscript 3
- K plus A L N which becomes K N plus A L
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because the reaction of potassium with aluminum nitrate forms K N O subscript 3 and A L, the formula for aluminum nitrate is A L left paren N O subscript 3 right paren subscript 3, the formula for potassium nitrate is K N O subscript 3 and the equation is balanced with an equal number of each type of atom on the right and left sides of the equation (i.e., three K atoms, one Al atom, three N atoms, and nine O atoms). Option B is incorrect because the formula for aluminum nitrate is not correctly represented. Option C is incorrect because the incorrect products are formed, and the formula for aluminum nitrate is not correctly represented. Option D is incorrect because the incorrect products are formed, and the compound formulas are not correctly represented.
2 C subscript 2 H subscript 6 left paren g right paren plus 7 O subscript 2 left paren g right paren which becomes 4 CO subscript 2 left paren g right paren plus 6 H subscript 2 O left paren g right paren
21. According to the balanced equation above, what is the maximum mass of CO subscript 2 that can be produced if 3.00 mol of C subscript 2 H subscript 6 left paren g right paren and 160.0 g of oxygen are available?
- 2.86 g
- 6.00 g
- 126 g
- 264 g
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because the mass that is produced is 126 g. The oxygen is the limiting reagent. 160 g oxygen is 5 moles of O subscript 2 . Based on the balanced equation, 5 moles of O subscript 2 can completely react with 1.42 moles of C subscript 2 H subscript 6. left paren 5 O subscript 2 times start fraction 2 C subscript 2 H subscript 6 over 7 O subscript 2 end fraction equals 1.42 mol C subscript 2 H subscript 6 right paren. So the 3 moles of C subscript 2 H subscript 6 is in excess. Hence, the maximum mass of CO subscript 2 that can be produced is 126 g (to three significant figures), calculated as follows: 5 mol O subscript 2 times start fraction 4 mol CO subscript 2 over 7 mol O subscript 2 end fraction times start fraction 44 g CO subscript 2 over 1 mol CO subscript 2 end fraction equals 126 g CO subscript 2. Option A is incorrect because 2.86 is the number of moles of CO subscript 2 produced, not the mass produced. Option B is incorrect because 6.0 is neither the number of moles nor the mass of the product produced. Option D is incorrect because 264 is the mass of CO subscript 2 that would be produced if all the C subscript 2 H subscript 6 was consumed, but there is insufficient oxygen to do that.
Competency 010—The teacher understands types and properties of solutions.
22. If 23.8 g of MgCl subscript 2 is completely dissolved in water, forming a 500.0 mL solution, which of the following is the concentration of Cl negative in the solution? (Assume molar mass of MgCl subscript 2 is 95.2 g.)
- 0.050 M
- 0.250 M
- 0.500 M
- 1.00 M
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because concentration of Cl negative is 1.00 M, calculated as follows:
Since 500 mL = 0.5 L, then
23.8 g M G C L subscript 2 over 0.5 L times 1 mol M G C L subscript 2 over 95.2 g M G C L subscript 2 times 2 C L negative over 1 M G C L subscript 2 equals 1.00 M the fraction.
Option A is incorrect because 0.050 does not correctly give the concentration of Cl negative expressed in mol/L; it is the approximate concentration of MgCl subscript 2 expressed in g/mL. Option B is incorrect because 0.250 does not correctly give the concentration of Cl negative expressed in mol/L; it is the total number of moles of MgCl subscript 2 dissolved. Option C is incorrect because 0.500 does not correctly give the concentration of Cl negative expressed in mol/L; it is the number of moles of MgCl subscript 2 per liter.
23. Of the following solutions, which has the lowest freezing point?
- 0.5 m CaCl subscript 2 left paren aq right paren
- 0.5 m NaCl left paren aq right paren
- 0.5 m NaNO subscript 3 left paren aq right paren
- 0.5 m HCl left paren aq right paren
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because 0.5 m CaCl subscript 2 dissociates into 3 ions in solution left paren CaCl subscript 2 yields Ca squared positive plus 2 Cl negative right paren, and the number of particles in solution is proportional to the freezing point depression. Option B is incorrect because 0.5 m NaCl dissociates into 2 ions in solution left paren NaCl yields Na positive plus Cl negative right paren and has fewer particles in solution than 0.5 m CaCl subscript 2. Hence, its freezing point depression is less than that of 0.5 m CaCl subscript 2. Option C is incorrect because 0.5 m NaNO subscript 3 dissociates into 2 ions in solution left paren NaNO subscript 3 yields Na positive plus NO subscript 3 negative right paren and has fewer particles in solution than does 0.5 m CaCl subscript 2. Hence, its freezing point depression is less than that of 0.5 m CaCl subscript 2. Option D is incorrect because 0.5 m HCl dissociates into 2 ions in solution left paren HCl yields H positive plus Cl negative right paren and has fewer particles in solution than does 0.5 m CaCl subscript 2. Hence, its freezing point depression is less than that of 0.5 m CaCl subscript 2.
24. Which of the following actions will change the solubility of a solid compound in water?
- Stirring the water vigorously
- Using a powder solid rather than a chunk solid
- Adding more water
- Changing the temperature of the water
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because solubility is defined as the maximum mass of a solid that can dissolve in 100 mL of a solution at a particular temperature. Changing the temperature will increase or decrease the solubility (maximum mass that can dissolve in 100 mL). Option A is incorrect because stirring only increases the rate of dissolving and has no effect on the solubility (maximum mass that can dissolve in 100 mL). Option B is incorrect because using powder instead of chunks only increases the rate of dissolving and has no effect on the solubility (maximum mass that can dissolve in 100 mL). Option C is incorrect because adding water can increase the total mass that dissolves, but it has no effect on the solubility (maximum mass that can dissolve in 100 mL). The maximum possible concentration of the solution is the same.
Competency 011—The teacher understands energy transformations that occur in physical and chemical processes.
25. Which of the following is an exothermic process?
- Bonds breaking in a chemical reaction: H subscript 3 C single bond CH subscript 3 which becomes 2 times CH subscript 3
- Water evaporating from a puddle: H subscript 2 O left paren l right paren which becomes H subscript 2 O left paren g right paren
- Frost forming on the ground from water vapor in the air: H subscript 2 O left paren g right paren which becomes H subscript 2 O left paren s right paren
- Ionization of gaseous sodium atoms: Na left paren g right paren which becomes Na positive left paren g right paren
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because energy is released (exothermic) when the gaseous water molecules come together to form solid H subscript 2 O in a crystal structure (frost). The potential energy is higher when the molecules are far apart in the gaseous state, and the potential energy is reduced when the molecules are close together in the solid state. Option A is incorrect because the input of energy is required (endothermic) to break a bond between carbon atoms. Option B is incorrect because the input of energy is required (endothermic) to change liquid water to gaseous water in which the molecules are much farther apart (higher potential energy). Option D is incorrect because the removal of an electron from sodium to form an ion requires the input of energy (endothermic).
26. A 50.0 gram sample of a metal at 100.0 degrees C is dropped into a beaker of 25.0 degrees C water. The water warms up and the metal cools down. After thermal equilibrium was reached at 30.0 degrees C, it was determined that 4,000.0 J of energy was absorbed by the water. What is the specific heat capacity of the metal?
- 1.75 J g superscript negative 1 degree C superscript negative 1
- 1.14 J g superscript negative 1 degree C superscript negative 1
- 0.875 J g superscript negative 1 degree C superscript negative 1
- 0.570 J g superscript negative 1 degree C superscript negative 1
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because the specific heat capacity of the metal is 1.14 J g superscript negative 1 degree C superscript negative 1. The heat lost by the cooling metal is equal to the heat absorbed by the water and is found from
start fraction 4000 J over left paren 50.0 g right paren left paren 70.0 degrees C right paren end fraction equals 1.14 J g to the power of negative 1 1 degrees C to the power of negative 1. Options A, B, and C are incorrect.
Domain III—Chemical Reactions
Competency 012—The teacher understands chemical kinetics and equilibrium.
A plus 2 B which becomes 2 C
27. For the reaction represented above, the initial reaction rate is determined using initial concentrations: [A] = 1 M, [B] = 1 M, and [C] = 0. When the reaction is run again using initial concentrations: [A] = 1 M, [B] = 2 M, and [C] = 0, the initial reaction rate was found to have doubled. Based on the data, which of the following could be the initial rate law?
- Rate = [A][B]
- Rate = [A][B] squared
- Rate = [A] squared [B] squared
- Rate = [A][B] squared over [C] squared
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because for the initial rate law Rate = [A][B], the initial reaction rate doubles when [B] doubles, calculated as follows: start fraction Rate 2 over Rate 1 end fraction equals start fraction left bracket A right bracket subscript 2 over left bracket A right bracket subscript 1 end fraction times start fraction left bracket B right bracket subscript 2 over left bracket B right bracket subscript 1 end fraction equals start fraction left paren 1 M right paren over left paren 1 M right paren end fraction times start fraction left paren 2 M right paren over left paren 1 M right paren end fraction equals 2.
Option B is incorrect because for the initial rate law Rate = [A][B] squared, the initial reaction rate increases fourfold when [B] doubles, calculated as follows: start fraction Rate 2 over Rate 1 end fraction equals start fraction left bracket A right bracket subscript 2 over left bracket A right bracket subscript 1 end fraction times start fraction left bracket B right bracket superscript 2 base subscript 2 over left bracket B right bracket superscript 2 over base subscript 1 end fraction equals start fraction left paren 1 M right paren over left paren 1 M right paren end fraction times start fraction left paren 2 M right paren squared over left paren 1 M right paren squared end fraction equals 4.
Option C is incorrect because for the initial rate law Rate = [A] squared [B] squared, the initial reaction rate increases fourfold when [B] doubles, calculated as follows: start fraction Rate 2 over Rate 1 end fraction equals start fraction left bracket A right bracket superscript 2 over base subscript 2 over left bracket A right bracket superscript 2 over base subscript 1 end fraction times start fraction left bracket B right bracket superscript 2 base subscript 2 over left bracket B right bracket superscript 2 over base subscript 1 end fraction equals start fraction left paren 1 M right paren squared over left paren 1 M right paren squared end fraction times start fraction left paren 2 M right paren squared over left paren 1 M right paren squared end fraction equals 4.
Option D is incorrect because for the initial rate law Rate = left bracket A right bracket left bracket B right bracket squared over left bracket C right bracket squared, again the initial reaction rate increases fourfold when [B] doubles, due to the [B] squared term in the rate law.
k = A e to the power of negative fraction E subscript a over RT
28. For some chemical reactions, the rate constant follows the Arrhenius expression, given above. What does the E subscript a term represent in the Arrhenius expression?
- The change in enthalpy for the reaction
- The change in Gibbs energy for the reaction
- The change in entropy for the reaction
- The activation energy for the reaction
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because E subscript a represents the activation energy. Option A is incorrect because E subscript a does not represent the change in enthalpy for the reaction left paren Delta H subscript rxn right paren. Option B is incorrect because E subscript a does not represent the change in Gibbs energy for the reaction left paren Delta G subscript rxn right paren. Option C is incorrect because E subscript a does not represent the change in entropy for the reaction left paren Delta S subscript rxn right paren.
A subscript 2 left paren g right paren plus 2 B subscript 2 left paren g right paren in equilibrium with 2 B subscript 2 A left paren g right paren
29. Which of the following is the expression for the equilibrium constant, in terms of concentration, for the equilibrium represented above?
- K subscript c equals left bracket B subscript 2 A right bracket squared over left bracket A subscript 2 right bracket left bracket B subscript 2 right bracket squared
- K subscript c equals left bracket A subscript 2 right bracket left bracket B subscript 2 right bracket squared over left bracket B subscript 2 A right bracket squared
- K subscript c equals left bracket 2 B subscript 2 A right bracket squared over left bracket A subscript 2 right bracket left bracket 2 B subscript 2 right bracket squared
- K subscript c equals left bracket A subscript 2 right bracket left bracket 2 B subscript 2 right bracket squared over left bracket 2 B subscript 2 A right bracket squared
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because the equilibrium constant expression should have the concentration of the product in the numerator, raised to the power that corresponds to the coefficient for the product in the balanced equation (in this case the coefficient is 2). And the concentrations of the reactants should be in the denominator, each raised to the power that corresponds to the coefficient for that reactant in the balanced equation (in this case the coefficients are 1 for A subscript 2 and 2 for B subscript 2). Options B, C, and D are incorrect.
Competency 013—The teacher understands acids, bases and their reactions.
30. Which of the following mixtures will form a buffer solution?
- 50 mL of 0.01 M KCl left paren aq right paren and 50 mL of 0.01 M KOH left paren aq right paren
- 50 mL of 0.01 M KCl left paren aq right paren and 50 mL of 0.01 M HCl left paren aq right paren
- 50 mL of 0.01 M KOH left paren aq right paren and 50 mL of 0.01 M HCl left paren aq right parenl
- 50 mL of 0.01 M CH subscript 3 COOH left paren aq right paren and 50 mL of 0.01 M CH subscript 3 COOK left paren aq right paren
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because a 1:1 mixture of a weak acid (CH subscript 3 COOH) and the salt of a weak acid (CH subscript 3 COOK) will form a buffer solution. There is an equilibrium involving the CH subscript 3 COOH and the CH subscript 3 COO negative. Small additions of either acid or base will not result in a pH change based on the equilibrium. Option A is incorrect because a mixture of a neutral salt (KCl) and a strong base (KOH) will not form a buffer solution. Option B is incorrect because a mixture of a neutral salt (KCl) and a strong acid (HCl) will not form a buffer solution. Option C is incorrect because a mixture of a strong base (KOH) and a strong acid (HCl) will not form a buffer solution.
31. What is the pH of 0.001 M NaOH left paren aq right paren at 298 K?
- 1.0
- 3.0
- 8.0
- 11.0
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because pH = 11.0 for 0.001 M NaOH solution. For an aqueous solution, the pH is equal to negative log left bracket H positive right bracket. And at 298 K,
left bracket H positive right bracket equals start fraction 1 times 10 to the power of negative 14 over left bracket OH negative right bracket end fraction equals start fraction 1 times 10 to the power of negative 14 over left paren 0.0001 right paren end fraction equals 1 times 10 to the power of negative 11 M. So pH = 11.0, based on the following calculation: since pH equals negative log left bracket H positive right bracket, then
pH equals negative log left paren 1 times 10 to the power of negative 11 right paren equals negative left paren negative 11 right paren equals 11.0. Options A, B and C are incorrect.
32. A 1 M aqueous solution of an acid HA with pH = 6.0 is titrated with a 0.1 M NaOH solution. If the pH = 10.0 at the equivalence point, which of the following is most likely true about the acid HA?
- HA is a strong acid
- HA is a weak acid
- HA is a strong electrolyte
- HA is chemically inert
- Enter to expand or collapse answer.Answer expanded
- Option B is correct because when a weak acid is titrated by a strong base such as NaOH, the equivalence point is not at pH = 7.0, but instead will be in the basic range (in this case, pH = 10.0). This is explained by the hydrolysis of the salt that forms, NaA. Because A negative is a conjugate base of a weak acid, it will react with water to form some HA and OH negative left paren A negative plus H subscript 2 O in equilibrium with HA plus OH negative right paren. The pH at the equivalence point will be related to the K subscript a of the acid. Option A is incorrect because HA is not a strong acid, as indicated by the pH at the equivalence point. Option C is incorrect because HA is a weak acid, as indicated by the pH at the equivalence point. Hence, it cannot be a strong electrolyte because weak acids are those that do not dissociate completely in water. Strong electrolytes are compounds that completely dissociate into ions in solution. Option D is incorrect because HA is reacting with the NaOH during the titration, as evidenced by the change in pH from 6.0 to 10.0. Hence, it is not chemically inert.
Competency 014—The teacher understands oxidation and reduction reactions.
AgNO subscript 3 left paren aq right paren + NaCl left paren s right paren) in equilibrium with AgCl left paren s right paren plus Na positive left paren aq right paren plus NO subscript 3 negative left paren aq right paren
33. Which of the following is true about the reaction represented above?
- Silver is reduced.
- Sodium is oxidized.
- Chlorine is reduced.
- No oxidation or reduction is taking place in the reaction.
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because none of the species have a change in oxidation state during the reaction; no oxidation or reduction is taking place. Option A is incorrect because silver has an oxidation state of +1 in both reactants and products; hence, is not being reduced. Option B is incorrect because sodium has an oxidation state of +1 in both reactants and products; hence, is not being oxidized. Option C is incorrect because chlorine has an oxidation state of negative 1 in both reactants and products; hence, is not being reduced.
34. If 5.00 amperes are provided for 10.0 hours in an electroplating experiment, which of the following is the maximum amount of Cu squared positive that can be plated out of 3.00 L of a 2.00 M CuSO subscript 4 solution? (Assume F = 96,500 C/mol.)
- 0.933 mol Cu
- 1.87 mol Cu
- 3.73 mol Cu
- 6.00 mol Cu
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because the maximum amount of copper that can be plated out, based on the current provided, is 0.933 mol Cu, calculated as follows:
5 amperes equals 5 Coulombs/second equals 5 C/s and
start fraction 5 C over s end fraction times 10 hr times start fraction 60 min over 1 hr end fraction times start fraction 60 s over 1 min end fraction equals 180,000 C and
180,000 C times start fraction 1 mol e negative over 96,500 C end fraction equals 1.865 mol e negative and 1.865 mol e negative times start fraction 1 mol Cu squared positive over 2 mol e negative end fraction equals 0.933 mol Cu. Option B is incorrect because 1.87 mol of Cu is twice the amount that is produced and does not account for the fact that 2 moles of electrons are needed to plate out 1 mole of Cu. Option C is incorrect because 3.73 mol is four times the amount that is produced. Option D is incorrect because 6.00 mol of Cu is far larger than the amount that can be produced and is equal to all of the Cu that is contained in the 3.00 L solution.
Competency 015—The teacher understands nuclear fission, nuclear fusion and nuclear reactions.
35. Which of the following nuclear processes is an example of an alpha emission?
- B I with a mass number of 215 becomes P O with a mass number of 215
- U with a mass number of 240 becomes N P with a mass number of 240
- R N with a mass number of 222 becomes P O with a mass number of 218
- K with a mass number of 40 becomes A R with a mass number of 40
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because an alpha particle is emitted in the process: R N with a mass number of 222 becomes P O with a mass number of 218 left paren RN with a mass number of 222 and an atomic number of 86 becomes P O with a mass number of 218 and an atomic number of 84 plus H E with a mass number of 4 and an atomic number of 2 right paren
Option A is incorrect because a negative beta emission is taking place in which an electron is emitted in the process: B I with a mass number of 215 becomes P O with a mass number of 215 left paren B I with a mass number of 215 and an atomic number of 83 becomes P O with a mass number of 215 and an atomic number of 84 plus e with a mass number of 0 and an atomic number of negative 1 right paren
Option B is incorrect because a negative beta emission is taking place in which an electron is emitted in the process: U with a mass number of 240 and an atomic number of 92 becomes N P with a mass number of 240 and atomic number of 93 plus e with a mass number of 0 and an atomic number of negative 1 right paren
Option D is incorrect because a positive beta emission is taking place in which a positron is emitted in the process: K with a mass number of 40 and an atomic number of 19 becomes A R with a mass number of 40 and atomic number of 18 plus e with a mass number of 0 and an atomic number of positive 1 right paren
36. The half-life of a radioactive isotope of hydrogen is 12.3 years. If the original sample of the isotope is 16 micrograms, which TWO of the following statements are true after 25 years elapse?
- A little more than 8.0 micrograms of the isotope will remain.
- A little less than 4.0 micrograms of the isotope will remain.
- More than two half-lives have elapsed.
- Although radioactive decay occurred, the mass of the isotope will still be 16 micrograms.
- Enter to expand or collapse answer.Answer expanded
- Options B and C are correct because after 25 years, the mass of the isotope that will remain is 3.9 micrograms, which is a little less than 4.0 micrograms. Since the half-life is 12.3 years, 25 years is more than two half-lives. If only two half-lives had elapsed (24.6 years), then the mass of isotope remaining would have been 4.0 micrograms:
1 half times 1 half times 16 micrograms equals 4 micrograms.
The actual mass remaining after 25 years elapse can be calculated using the following steps. Based on the half-life, the decay rate constant can be found from
negative kt equals 0.693 over 12.3 years.
Based on first-order decay kinetics, the amount of isotope remaining (x) is found from
start fraction ln x over ln 16 micrograms end fraction equals negative kt equals 0 start fraction 0.693 over 12.3 years end fraction times 25 years.
Solving this equation for x yields 3.9 micrograms of isotope remaining.
Options A and D are incorrect.
Domain IV—Science Learning, Instruction and Assessment
Competency 016—The teacher understands research-based theoretical and practical knowledge about teaching science, how students learn science and the role of scientific inquiry in science instruction.
37. Which of the following activities is most appropriate after a short lesson on the significance of oxidation numbers?
- The students attempt to balance a simple redox reaction
- The teacher presents a demonstration involving a combustion reaction
- The students perform an acid-base titration in the lab
- The students determine the number of neutrons in one atom of iodine-131 based on information from the periodic table
- Enter to expand or collapse answer.Answer expanded
- Option A is correct because the oxidation number of the elements in the various species in the reaction must be known in order to correctly balance a redox (oxidation-reduction) reaction. In addition to trying to balance the number of atoms on both sides of the equation, the students must also balance the increase and decrease in oxidation numbers that take place, and various species gain or lose electrons. Option B is incorrect because although a combustion reaction may involve an oxidation and reduction, the demonstration would not illustrate the use of oxidation numbers. Option C is incorrect because an acid-base titration may or may not involve an oxidation-reduction, but the titration would not illustrate the use of oxidation numbers. Option D is incorrect because the number of neutrons in iodine-131 can be deduced by finding the atomic number of iodine on the periodic table. The oxidation state of iodine is not related to this determination.
38. Which of the following is an element of inquiry-based science instruction?
- A teacher-led question and answer session
- A video presentation of science principles to be included in a unit of study
- A student forming a hypothesis prior to a lab activity
- A student writing a report after researching information on the Internet
- Enter to expand or collapse answer.Answer expanded
- Option C is correct because inquiry-based learning does involve students proposing a hypothesis prior to designing an experiment to test the hypothesis. Option A is incorrect because a teacher asking questions is important, but it is not an element of inquiry-based science instruction. Option B is incorrect because videos can be helpful, but they are not elements of inquiry-based learning. Option D is incorrect because writing reports can have value, but it is not an element of inquiry-based learning.
Competency 017—The teacher knows how to monitor and assess science learning in laboratory, field and classroom settings.
39. Of the following, which is the most appropriate way to assess a student's understanding of preparing a 1 M HCl solution from a 6 M HCl solution?
- Short quiz
- Lab report
- Summative assessment
- Performance assessment
- Enter to expand or collapse answer.Answer expanded
- Option D is correct because an assessment of the students preparing the solution in the lab is the best way to assess their understanding of the theory, their implementation of the theory and their technique. This approach is called a performance assessment. Option A is incorrect because a short quiz would assess only some theoretical understanding of how to prepare solutions by dilution, but it will not assess the students' ability to implement the theory or their technique. Option B is incorrect because a report can assess only how well students explain what should be done, but it will not assess their ability to implement the theory or their technique. Option C is incorrect because a summative assessment will assess only some theoretical understanding of how to prepare solutions by dilution, but it will not assess students' ability to implement the theory or their technique.
40. When teaching a complex unit it is important to frequently monitor and assess the students' understanding of the concepts throughout the unit by doing which of the following?
- Facilitating a class discussion while monitoring responses and questions
- Having students conduct frequent lab exercises with full lab reports
- Administering a series of short quizzes periodically throughout the unit
- Assigning a multistep research project that is due at the end of the unit
- Enter to expand or collapse answer.Answer expanded
- Options A and C are correct because they will effectively assess the students' understanding of the concepts throughout the unit. Option B is incorrect because frequent full lab reports will be time-consuming and will assess much more than what is needed. Option D is incorrect because the feedback to the teacher will not be available until the end of the unit and will not provide the frequent monitoring of understanding that was desired.
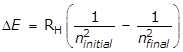
![Rate 2 over Rate 1 = [A] subscript 2 over [A] subscript 1 times [B] subscript 2 over [B] subscript 1 equals (1 M) over (1 M) times (2 M) over (1 M) = 2](images\Questions\240_27_02.png)
![Rate 2 over Rate 1 = [A] subscript 2 over [A] subscript 1 times [B] superscript 2 over base subscript 2 over [B] superscript 2 over base subscript 1 equals (1 M) over (1 M) times (2 M) squared over (1 M) squared = 4](images\Questions\240_27_03.png)
![Rate 2 over Rate 1 = [A] superscript 2 over base subscript 2 over [A] superscript 2 over base subscript 1 times [B] superscript 2 over base subscript 2 over [B] superscript 2 over base subscript 1 equals (1 M) squared over (1 M) squared times (2 M) squared over (1 M) squared = 4](images\Questions\240_27_04.png)
![[A][B] squared over [C] squared](images\Questions\240_27_05.png)